During process development or plant operation it is often necessary to estimate energy of reaction based on chemical formulae representation alone. This heat of decomposition represents the potential energy that can be released and is therefore a measure of explosion potential. One can therefore envision that estimation of heats reaction based on chemical similarities should be possible similar to Benson’s group additivity for estimating heats of formation.
I have compiled heats of polymerization and heats of formation from a variety of sources. You can use the functional groups from these tables to estimate heats of reaction for your system of interest.
Heats of Polymerization
| Functional Group | Structure | Mean heat of reaction | Min…..Max. |
|---|---|---|---|
| kcal/mol | kcal/mol | ||
| Olefins | -C=C- | 20 | |
| Acetylenes | -C≡C- | 30 | |
| -C=O- | 5 | ||
| -C=N- | 1.4 | ||
| -C≡N- | 7 | ||
| Sulfolane | -S=O | 7 | |
| -C=S- | 2 |
Heats of Reaction - Carbon Containing Groups
| Functional Group | Structure | Mean -∆H | Min…..Max. |
|---|---|---|---|
| Epoxide | -C-C- | 20 | 17-24 |
| Hydro peroxide | -OOH | 60 | 55-67 |
| Dialkyl peroxides | R-O-O-R | 50 | 43-55 |
| Peroxyacids | -C(=O)OOH | 63 | 57-69 |
| Diacyl peroxy | -C(=O)-O-O-C(=O) | 75 | 55-86 |
| Hydrazine | -N-N- | 18 | 15-21 |
| Azo | -N=N- | 50 | 28-185 |
| Diazo | -N2+ | 40 | 38-43 |
| >C=N≡N | 50 | 41-57 | |
| Azide | N3 | 53 | 48-58 |
Heats of Reaction - Nitrogen Containing Groups
| Functional Group | Structure | Mean -∆H | Min...Max |
|---|---|---|---|
| In a ring | -N-N- | 24 | 4-72 |
| Imidazole | N-C-N | 31 | 10-51 |
| Tetrazole | 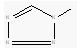 |
50 | 33-84 |
| Trizene | -N=N-N< | 50 | 33-84 |
| C-Nitro | -C-NO2 | 75 | 60-130 |
| O-Nitro | -O-NO2 | 110 | 105-115 |
| N-Nitro | -N-NO2 | 100 | 93-108 |
| Nitroso | -N=O- | 48 | 33-70 |
| N-Oxide | -N-O- | 34 | 21-51 |
| Oxime | >N-OH | 34 | 25-68 |
| Oxazole | N-C-O | 19 | 8-43 |
| Isocyanate | -N=C=O | 15 | 12-18 |
Examples
1. Heat of polymerization for Styrene
To predict heat of polymerization I will use –C=C-.
Mean – 20 kcal/mol
Min – 12 kcal/mol
Max – 22 kcal/mol
Thus we estimate heat of polymerization of Styrene to be 217 cal/g [Range 130-239 cal/g]. The experimental heat of reaction for styrene is 151 cal/g.
2. Heat of reaction for Propylene oxide
To predict heat of polymerization I will use value for epoxide group.
Mean – 20 kcal/mol
Min – 17 kcal/mol
Max – 24 kcal/mol
Thus we estimate heat of polymerization of propylene oxide to be 343 cal/g [Range 292-412 cal/g]. The experimental heat of reaction for styrene is 266 cal/g.
To test the values in the two tables, I predicted reaction energies for 105 compounds.
For the 105 compound test set the average absolute error was 87 cal/g. I therefore believe that you can predict heats of reactions within 100 cal/g using the proposed values.
Knowing the heat of reaction for a compound gives you an idea of its explosion potential. As a rule of thumb, if the heat of reaction is greater than 750 cal/g, the compound is packing high energy.